High Soil pH: Understanding Plant Nutrition in Cranberries
Senay Ugur, Emily Hahn, Jyostna Devi Mura
In 2020, we conducted a preliminary study and found a significant effect of high soil pH (pH~ 7.2) both on plant nutrient absorption and on fruit size. A 40 to 60% fruit size reduction was observed in high soil pH (pH ~ 7.2) plots compared to optimum soil pH (pH ~5.0) beds. The fruit sampled from high soil pH plots had increased calcium (Ca) levels, and substantially decreased sodium (Na), iron (Fe), manganese (Mn), and zinc (Zn) levels compared to fruits from optimum soil pH beds. A similar trend in nutrient content was observed in leaves and stems. These preliminary results suggest that the cranberries under high soil pH are unable to take up some micronutrients from the soil, such as Na, Fe, Mn, and Zn—resulting in a significant decrease in fruit size.
Soil pH influences many soil characteristics such as microorganism activity, nutrient availability, and nutrient absorption. Most microorganisms have an optimum pH range for survival and function. Organic matter mineralization slows or stops at very acid or alkaline pH levels because of poor microbial activity related to bacteria. The availability and absorption of some plant nutrients are affected by soil pH, as shown in Figure 1. Soil pH has little impact on the availability of nitrogen (N), sulfur (S), and potassium (K). However, N stays as ammonium (NH4+) form at pH 4.2-5.5, which is ideal for cranberry growth. Under high soil pH, NH4+ is converted to nitrate (NO3-) form that is not as bioavailable as ammonium for cranberry to absorb.
On the other hand, phosphorus (P) is directly affected by soil pH. For example, phosphate tends to react with calcium (Ca) and magnesium (Mg) at alkaline pH. In acidic soil, it reacts with aluminum (Al) and iron (Fe) and becomes less soluble. As soil pH increases, many nutrients, like magnesium Mg and Ca, become increasingly available, disturbing the nutrient balance and causing toxicity.
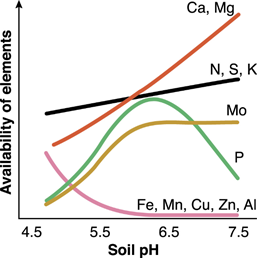
Soil pH can be adjusted for optimum cranberry growth. Soil pH is a measure of soil acidity or alkalinity. It depends on the hydrogen (H+) ion concentration in the soil solution; as H+ ions in the soil increase, the soil pH decreases. The pH of soil ranges from 0 to 14, with pH 7 being the neutral point. Soils with pH levels greater than 7 are considered alkaline, and those with pH less than 7 are referred to as acidic. Cranberries are best adapted to soils with pH between 4 and 6.
High pH (i.e. pH > 6) in a bed can be lowered by the application of elemental sulfur. Cranberry plants are adapted to tolerate high sulfur levels in the soil. Sulfur combines with water and oxygen to form sulfuric acid (H2SO4–) that has H+ ions which are released into the soil solution, thus lowering the pH. This process is time consuming since it involves a bacterium named Thiobacillus thiooxidans. This specific bacterium is always present in the soil at low levels. However, it takes time for the population to increase, depending on environmental conditions, for sufficient sulfur conversion to occur to lower soil pH. Alternatively, if the pH of a bed is very acidic (i.e. pH < 4), calcium (lime) can be used to raise the pH.
Recent visual observations this year have indicated that bud development in high pH soils is approximately 3 to 6 days behind that of optimum soils. Buds from optimum pH soils were at the hook stage (Image 1), while buds from high pH soils were either at bud elongation or early hook stage (Image 2) on June 4, 2021.

(Photo credits: Emily Hahn)
This year, we are conducting a study to replace micronutrient deficiencies in high pH soils by applying micronutrients as a foliar spray. We plan to evaluate the effect of foliar micronutrient application on cranberry fruit size both in high soil pH and optimum soil pH plants. Two distinct phases (hook and early fruit stage) were selected for foliar treatments. Soil, upright, and fruit nutrient data will be collected and compared before and after foliar application across pH treatments. In addition to foliar spray, we are studying soil microbiome amendments (bacteria and mycorrhizae) that can help maintain optimum pH in cranberry soils and nutrient absorption.
These studies will be conducted for two years, and we are looking forward to offering growers insight into whether the effects of extreme soil pH can be mitigated with foliar sprays.
This article was posted in Cranberry and tagged Cranberries, Emily Hahn, foliar micronutrients, Jyostna Devi Mura, overly high soil pH, Senay Ugur, Soil pH.
